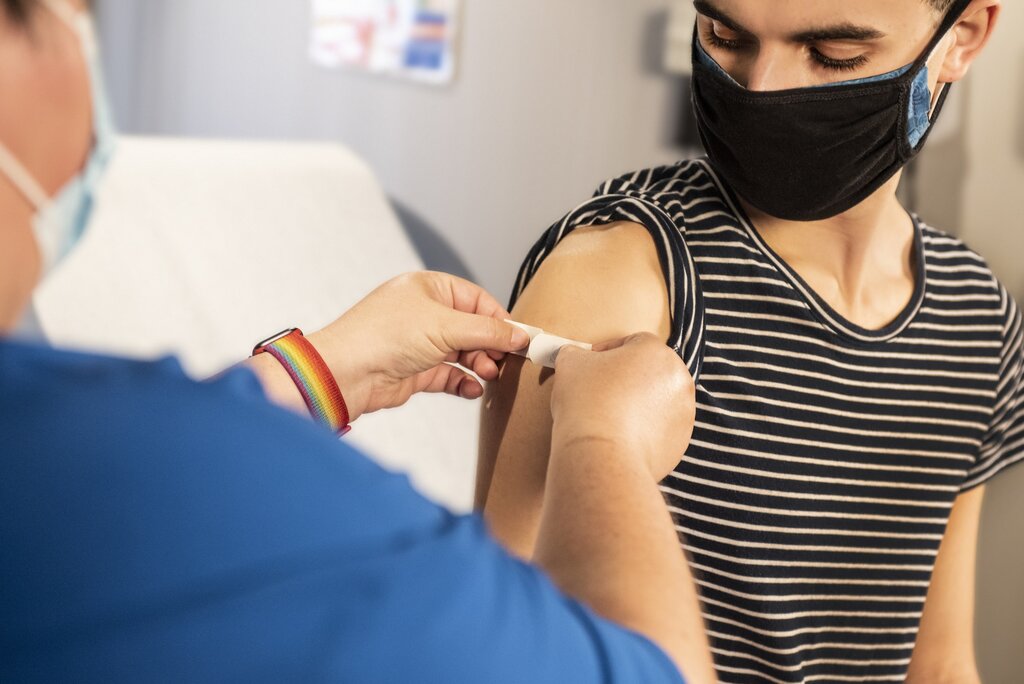
Novamax Inc. announced the U.S. Food and Drug Administration (FDA) granted its COVID-19 vaccine, NVX-CoV2373, emergency usage authorization (EUA) for youth ages 12 to 17 on Friday. Last month, the company received emergency approval for adults 18 and older.
FDA Commissioner Robert M. Califf, M.D. said: “Authorizing an additional COVID-19 vaccine expands the available options for the prevention of COVID-19, including the most severe outcomes that can occur such as hospitalization and death.” He assured the public the Novavax shot meets the FDA’s stringent requirements for safety, effectiveness, and manufacturing needed to support the EUA.
The newly authorized vaccine includes an adjuvant ingredient used in specific serums to help create a more robust immune response to help the body fight against the virus. These additives have been used in vaccines for more than 70 years. In the 1930s, 40s, and 50s, they were initially used in diphtheria and tetanus injections.
The CDC explains: “In all cases, vaccines containing adjuvants are tested for safety and effectiveness in clinical trials before they are licensed for use in the United States (in this case, an emergency use authorization), and they are continuously monitored by the CDC and FDA once they are approved.”
Two doses of Novamax’s NVX-CoV2373 primary series are given three to eight weeks apart. Moderately or severely immunocompromised individuals should receive the injections three weeks apart. It has not been authorized as a booster.
Novavax’s COVID-19 vaccine differs from Pfizer and Moderna’s mRNA shots. Instead, it was created using a conventional protein technology used in hepatitis B and HPV shots for decades.
Health experts encourage unvaccinated individuals to be vaccinated. However, since many do not want an mRNA-based injection, Novavax’s COVID-19 vaccine is an alternative to protect against severe coronavirus infection or death.
Written by Cathy Milne-Ware
Sources:
CDC: Novavax COVID-19, Adjuvanted Vaccine: Overview and Safety
FDA: Coronavirus (COVID-19) Update: FDA Authorizes Emergency Use of Novavax COVID-19 Vaccine, Adjuvanted
CDC: Adjuvants and Vaccines
CNBC News: FDA authorizes emergency use for Novavax Covid-19 vaccine for ages 12 to 17; by Annie Nova and Spencer Kimball
Featured and Top Image by CDC Courtesy of Unsplash – Creative Commons License
Inset Image by Maskmedicare Shop Courtesy of Unsplash – Creative Commons License