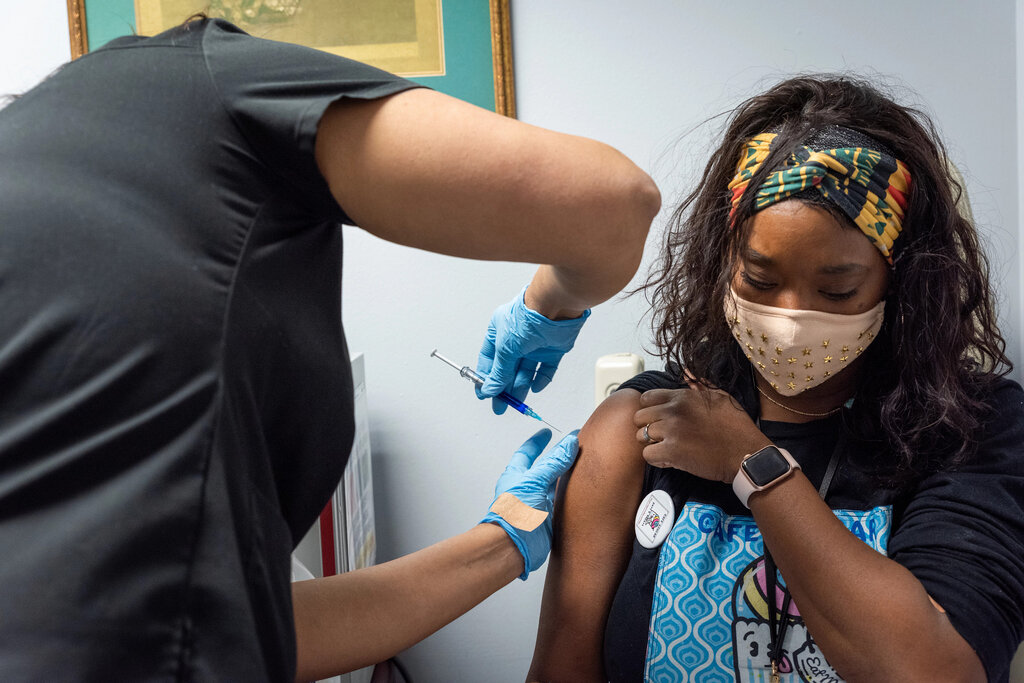
The Food and Drug Administration (FDA) has approved a new two-dose COVID vaccine called Novavax. This injection is based more on conventional protein technology used for decades in HPV and hepatitis B vaccines. The FDA authorized the new shots on July 13, 2022.
When the COVID-19 pandemic struck in 2020, Novavx was one of the original participants in the United States government’s race to develop a vaccine. They received $1.8 billion in taxpayer funding from Operation Warp Speed.
In early June the FDA’s committee of independent vaccine experts overwhelmingly voted in favor of the Novavax injection. Their decision came after an all-day public meeting where they weighed data on the vaccine’s safety and its ability at preventing illness from COVID.

The next step for the vaccine to become official the Centers for Disease Control and Prevention needs to sign off so healthcare providers and pharmacies can start administering the injections.
Novavax’s vaccine was reviewed for weeks before the FDA approved the shot. The Maryland biotech company toiled to produce its vaccine. Its clinical trial data was read much later than Moderna or Pfizer.
The FDA approved the new COVID vaccine after roughly 77% of adults ages 18 and older are already fully vaccinated. However, there are still 27 million adults in the U.S. who have yet to receive a single dose yet.
This COVID vaccine copies the virus spike outside human cells. The genetic code for the spike is placed into an insect virus that infects moth cells. This process produces copies that are then purified and extracted during manufacturing, according to CNBC. These finished spike copies are shot injected into people which induces an immune response against COVID-19.
The FDA feels that approving “an additional COVID-19 vaccine expands the available vaccine options for the prevention of” the virus. This includes possibly preventing “the most severe outcomes that can occur such as hospitalization and death,” stated FDA Commissioner Robert M. Califf, M.D.
He added that “vaccines remain the best preventive measure against severe disease caused by COVID-19.” Califf encouraged people who have been hesitant “to consider” getting their COVID vaccine.
Written by Sheena Robertson
Sources:
FDA: Coronavirus (COVID-19) Update: FDA Authorizes Emergency Use of Novavax COVID-19 Vaccine, Adjuvanted
Novavax: U.S. FDA Grants Emergency Use Authorization for Novavax COVID-19 Vaccine, Adjuvanted for Individuals Aged 18 and Over
CNBC: FDA authorizes Novavax Covid vaccine for adults as the first new shots in U.S. in more than a year
Image Courtesy of Novavax – Used with permission