A U.S. Food and Drug Administration (FDA) safety communication warns people not to use the COVID-19 Direct Antigen Rapid Test distributed by E25Bio as it is not authorized, cleared, or approved by the agency due to lack of transparency by the company. As a result, its distribution or use in the United States is not permitted.
The test may be marketed using its trade name, E25Bio SARS-CoV-2 Antigen Test Kit. However, since the company has not provided the FDA with sufficient data that demonstrate the test’s performance is accurate, the agency’s concern centers around the risk of false results. Additionally, the FDA learned the E25Bio COVID-19 Direct Antigen Rapid Test might have been sold directly to the public with instructions for collecting the sample that could result in grave injury when not administered by qualified professionals.
Injuries could include: damaging the tonsils/throat from the swab, potentially inducing vomiting, or pieces of the swab breaking off. In addition, the FDA warns that test swabs could be fragile and not appropriately designed for swabbing the nasopharyngeal (back of the throat) or oropharyngeal (middle part of the throat just beyond the mouth), which could cause a problem them to break.
No reports of injuries, adverse health consequences, or death associated with this test have been reported to the FDA. Nonetheless, the agency calls for health care providers, testing program organizers, the general public, including caregivers to use a different test instead. In addition, health officials are asking for any problems with the E25Bio COVID-19 Direct Antigen Rapid Test to the FDA, including inaccurate test results or injuries from self-swabbed nasopharyngeal or oropharyngeal be promptly reported (using this link).
FDA Explains COVID-19 Testing Basics
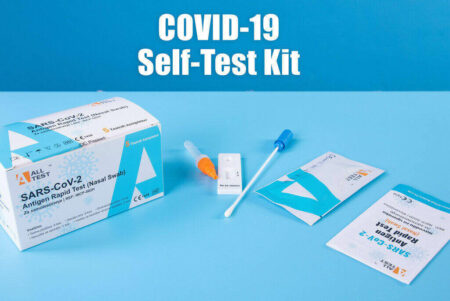
Diagnostic tests and antibody tests have different purposes. The first is basic; diagnostic tests detect an active COVID-19 infection present. There are two types of diagnostic tests, molecular, which detect genetic material, and antigen, which looks for specific proteins. Samples for these are typically collected with a nasal or throat swab or with saliva spit into a tube, according to the FDA.
The second type looks for antibodies in a person’s immune system produced in response to SARS-CoV-2, the virus that causes COVID-19. An antibody test should be used to detect active infection since antibodies can take days or weeks to develop after an infection starts. Moreover, it may remain in the person’s blood for several weeks after the symptoms are gone. Usually, these tests use a blood sample, either from a finger stick or blood is drawn a physician or other medical personnel.
Once a person is tested, the FDA states they need to quarantine and sequester themselves at home until they have their test results and follow a health care provider or public health expert’s advice.
There are numerous types of COVID-19 tests the FDA recommends for a person to collect their specimen:
- Home Collection: A sample is collected at home then transported to a laboratory for analysis. This test is provided with a prescription.
- Direct to Consumer (DTC): These home collection tests can be obtained without a prescription. The specimen is collected by the consumer and is shipped off for analysis.
- Over the Counter (OTC): Complete at-home sample collection and testing without a prescription.
To be sure to buy a test authorized by the FDA, the agency has lists for approved molecular and antigen tests.
Vaccinations, booster shots, and tests are available free of charge for everyone regardless of insurance or immigration status.
Written by Cathy Milne-Ware
Sources:
FDA: Do Not Use E25Bio COVID-19 Tests: FDA Safety Communication
FDA: In Vitro Diagnostics EUAs – Molecular Diagnostic Tests for SARS-CoV-2
The New York Times: At-Home COVID-19 Antigen Test Kits: Where to Buy and What You Should Know; by Tract Vence and Ellen Lee
Rolling Stone: These Rapid Covid Tests Are Selling Out Online As People Gather (Cautiously) Again
Becker’s Hospital Review: States ranked by COVID-19 hospitalization rates; by Gabrielle Masson
Featured and Top Image Courtesy of Mircea’s Pixabay Page – Creative Commons License
First Inset Image Courtesy of Alexandra_Koch’s Pixabay Page – Creative Commons License
Second Inset Image Courtesy of Jernej Furman’s Flickr Page – Creative Commons