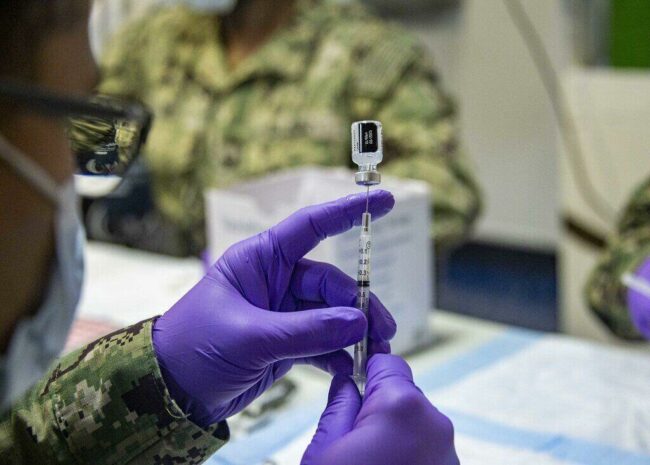
With the Delta variant of COVID-19 surging through much of the United States, the Food and Drug Administration (FDA) has sped up its timeline to fully approve Pfizer-BioNTech’s coronavirus vaccine. The agency hopes to complete the process by the start of September 2021.
Last week, President Joe Biden states that he fully expected the vaccine’s approval by early fall. However, the FDA’s unofficial deadline is Labor Day — Sept. 6, 2021 — or sooner, according to several people familiar with the agency’s plan. In a statement, the FDA said that its leaders acknowledge that approval might inspire more public confidence and they have “taken an all-hands-on-deck approach” to the work.
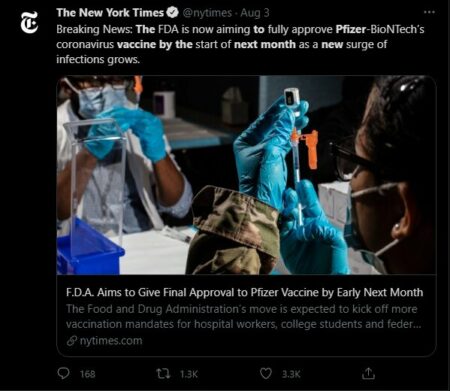
Last year the FDA granted emergency authorization for the Pfizer, Moderna, and Johnson & Johnson vaccines. Giving final approval to the Pfizer vaccine could increase the rates that individuals are being inoculated — especially with the Delta variant sharply increasing the number of new COVID-19 cases.
The Defense Department, San Francisco, and a number of hospitals and universities are expected to mandate inoculation once a COVID-19 vaccine is fully approved. Hopefully, the FDA’s final approval will help squash misinformation about the vaccine’s safety and clarify some legal issues about mandates.
According to The New York Times, the dean of the Brown University School of Public Health, Dr. Ashish K. Jha, said he has not “sensed a sense of urgency from the F.D.A. on full approval.”
And I find it baffling, given where we are as a country in terms of infections, hospitalizations and deaths.
Around 192 million U.S. citizens — 58 percent of the total population and 70 percent of the adults in the nation — have received at least one vaccine injection. Many individuals remain vulnerable to the ultra contagious, dominant Delta variant. According to The New York Times, the U.S. sees almost 86,000 new COVID-19 infections daily. This is an increase of 142 percent from two weeks ago.
The FDA has been evaluating data from clinical studies — which included tens of thousands of individuals — for each coronavirus vaccine. COVID-19 vaccines were approved for emergency use because the FDA felt their potential benefits outweighed the known and possible risks.
Written by Sheena Robertson
Source:
The New York Times: F.D.A. Aims to Give Final Approval to Pfizer Vaccine by Early Next Month; Sharon LaFraniere and Noah Weiland
U.S. Food & Drug Administration: Learn More About COVID-19 Vaccines From the FDA
Top and Featured Image Courtesy of U.S. Pacific Fleet’s Flickr Page – Creative Commons License